Lean Manufacturing Blog
by Jason Bullard, on Dec 6, 2024 6:57:40 PM
Practical applications for modern manufacturing solutions that fix real-world problems. A practical and straightforward explanation of how connected process control technologies can solve manufacturers’ problems. That doesn’t seem like too …
Read Storyby Jason Bullard, on Dec 6, 2024 3:24:25 PM
Practical applications for modern manufacturing solutions that fix real-world problems. Age-old challenges. Exciting new technologies. The intersection between those two opposing forces is a space rich with potential innovation, but …
Read Storyby Jason Bullard, on Nov 27, 2024 9:11:41 AM
Practical applications for modern manufacturing solutions that fix real-world problems Many manufacturers today find themselves at a pivot point: recognizing the power and potential of sophisticated new technologies, but hesitant …
Read Storyby Jason Bullard, on Nov 12, 2024 8:42:30 AM
Practical applications for modern manufacturing solutions that fix real-world problems. At a time when growing numbers of manufacturers are embracing an expanding ecosystem of powerful digital tools, increasingly sophisticated production …
Read Storyby Jason Bullard, on Sep 9, 2024 1:18:37 PM
The Future of Manufacturing: Part 3 Manufacturing is a field that has long been powered by creativity and innovation. From the steel-and-steam heritage of traditional production environments to the digital …
Read Storyby Jason Bullard, on Aug 14, 2024 4:07:47 PM
The Future of Manufacturing: Part 2 In the first blog in this series, we looked at the ways in which the future of manufacturing will be transformed by the supercharged …
Read Storyby Jason Bullard, on Jun 19, 2024 10:55:18 AM
During times of rapid change, it isn’t always easy to recognize the contours of an evolving tech landscape. For manufacturing professionals, it can be challenging to cut through the chaos …
Read Storyby Dan McKiernan, on May 6, 2024 1:05:14 PM
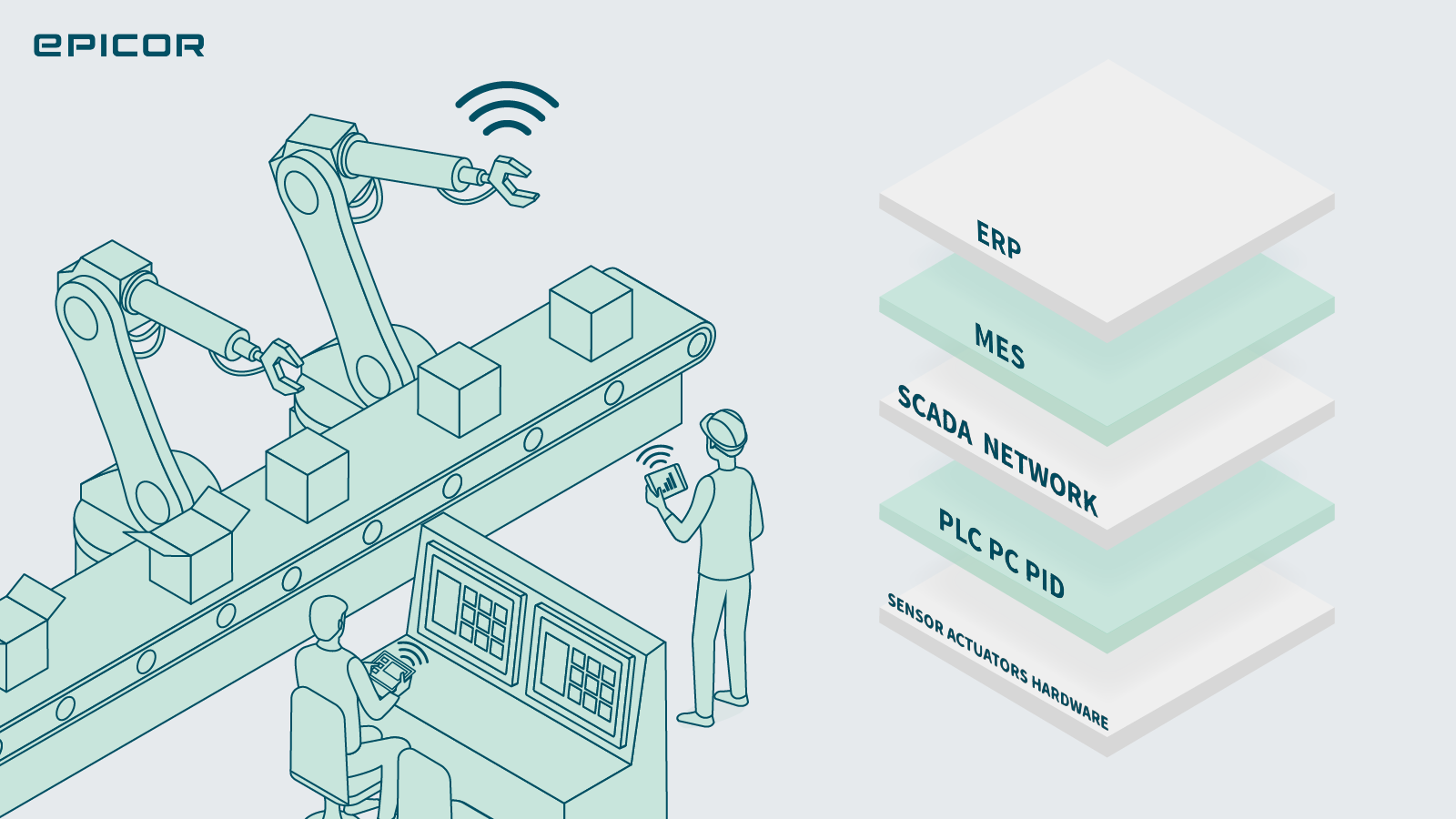
Integrating Enterprise Resource Planning (ERP) and Manufacturing Execution Systems (MES) on the plant floor can bring about significant benefits for an organization. These systems serve different but complementary functions, and …
Read Storyby Dan McKiernan, on Apr 23, 2024 4:36:24 PM
The critical importance of working with technology partners who walk the walk every day. In the manufacturing world, digital transformation is no longer a rarity. Having access to the powerful …
Read Storyby Dan McKiernan, on Apr 10, 2024 3:39:10 PM
Why personalized support can power your digital transformation If you, like growing numbers of manufacturers, are recognizing the game-changing potential of connected process control solutions and preparing to select the …
Read StoryThe Blog
Our team is a creative bunch that loves learning and pushing the limits to find the best solutions for today's lean manufacturers. Internal discussions at the office might range from new features that manufacturing process control software should have to machine learning, blockchain technology, or what the future of AR on the plant floor looks like. Check out our blog for opinions, news and trends that we find interesting and think you might too!
- Articles & Publications (7)
- Connected Process Control (33)
- Development Process (4)
- Digital Work Instructions (17)
- Educational Topics (9)
- Events (3)
- Food Industry (1)
- Industry 4.0 (32)
- Insider (9)
- Kitting (3)
- Lean Manufacturing (42)
- Manufacturing Culture (6)
- Manufacturing Execution System (1)
- Manufacturing Integrated Platform (7)
- Medical (2)
- News (28)
- Operator Training (2)
- Opinion (37)
- Partnerships (3)
- Press (4)
- Product Details (2)
- Quality Management (20)
- Team Building (2)
- Technology (23)
- Traceability (6)
- Training (1)
- Vehicle Assembly (3)
- February 2018 (6)
- May 2018 (6)
- September 2015 (5)
- November 2016 (4)
- May 2022 (4)
- January 2017 (3)
- March 2017 (3)
- June 2018 (3)
- February 2019 (3)
- November 2019 (3)
- February 2022 (3)
- June 2023 (3)
- July 2023 (3)
- November 2023 (3)
- February 2012 (2)
- December 2016 (2)
- March 2018 (2)
- April 2018 (2)
- March 2019 (2)
- July 2019 (2)
- September 2019 (2)
- January 2020 (2)
- January 2022 (2)
- March 2022 (2)
- May 2023 (2)
- August 2023 (2)
- October 2023 (2)
- December 2023 (2)
- January 2024 (2)
- March 2024 (2)
- April 2024 (2)
- November 2024 (2)
- December 2024 (2)
- August 2012 (1)
- November 2012 (1)
- March 2013 (1)
- June 2013 (1)
- September 2013 (1)
- November 2013 (1)
- December 2013 (1)
- February 2014 (1)
- July 2014 (1)
- September 2014 (1)
- February 2015 (1)
- March 2015 (1)
- August 2016 (1)
- October 2016 (1)
- February 2017 (1)
- October 2017 (1)
- July 2018 (1)
- August 2018 (1)
- October 2018 (1)
- January 2019 (1)
- April 2019 (1)
- May 2019 (1)
- June 2019 (1)
- October 2019 (1)
- March 2020 (1)
- June 2020 (1)
- October 2020 (1)
- October 2022 (1)
- April 2023 (1)
- February 2024 (1)
- May 2024 (1)
- June 2024 (1)
- August 2024 (1)
- September 2024 (1)



.png)